Single Use Systems
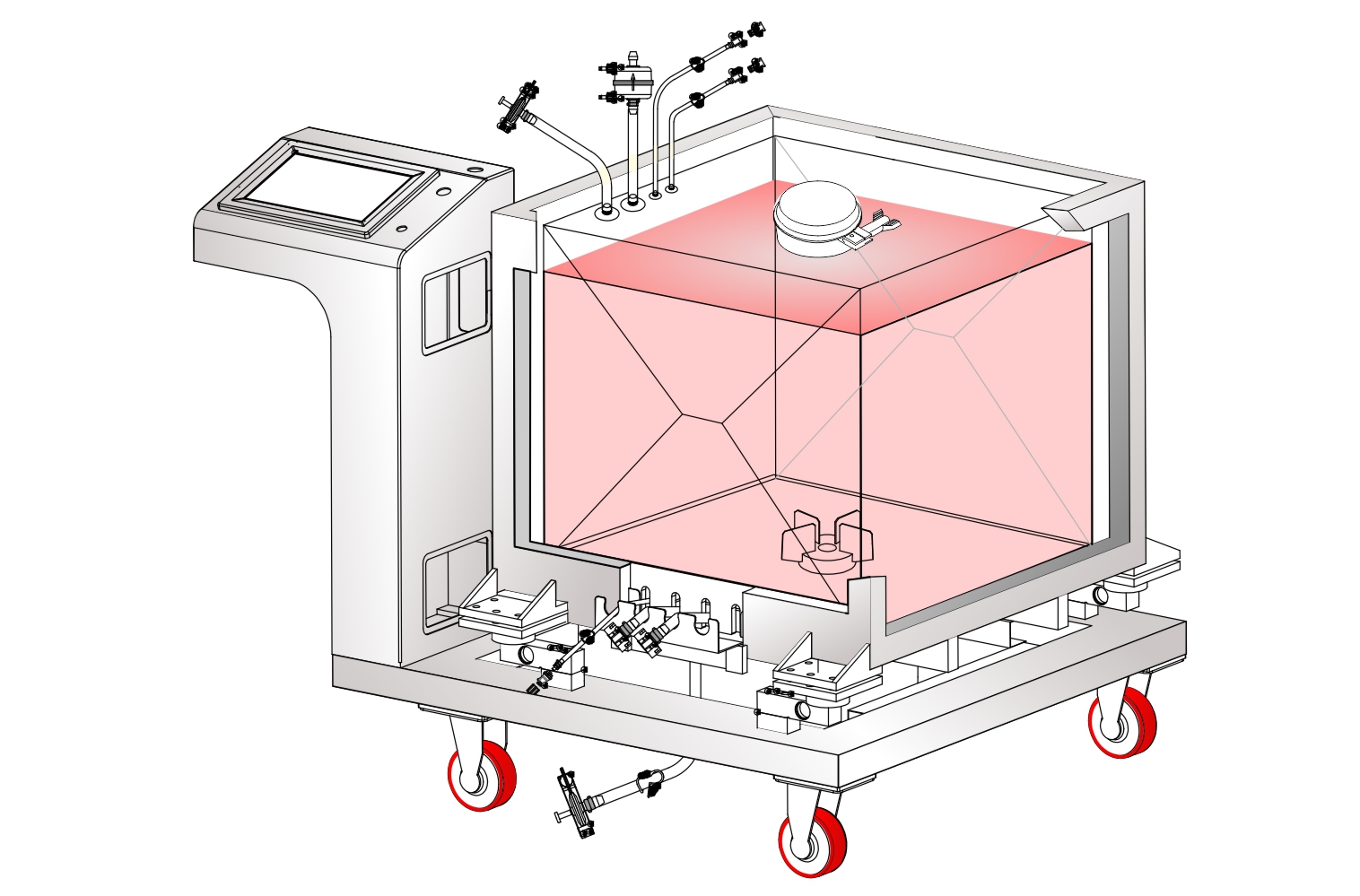
The biopharmaceutical industry is increasingly moving towards use of single use disposable systems for development as well as manufacture of a wide range of vaccines, therapeutic proteins and mAbs. Biopharmaceutical processes involve multiple steps with a multitude of process intermediates with different process conditions and objectives at each step. Single Use Systems (SUS) offer multiple advantages of reduced capital expenditure, reduced change over time and increased process flexibility while doing away with expensive and time consuming CIP/ SIP procedures and validation requirements associated with reusable stainless steel systems.
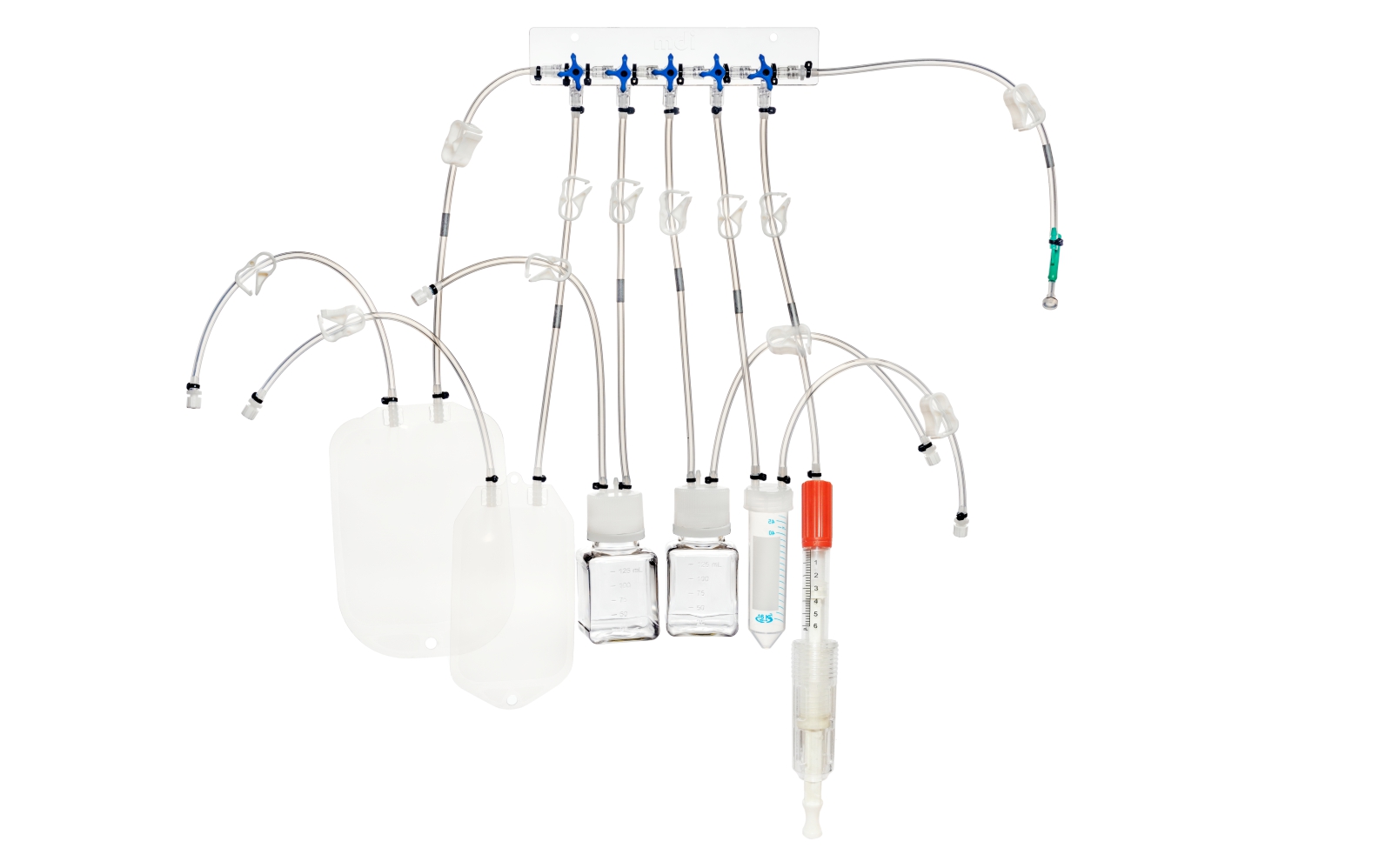
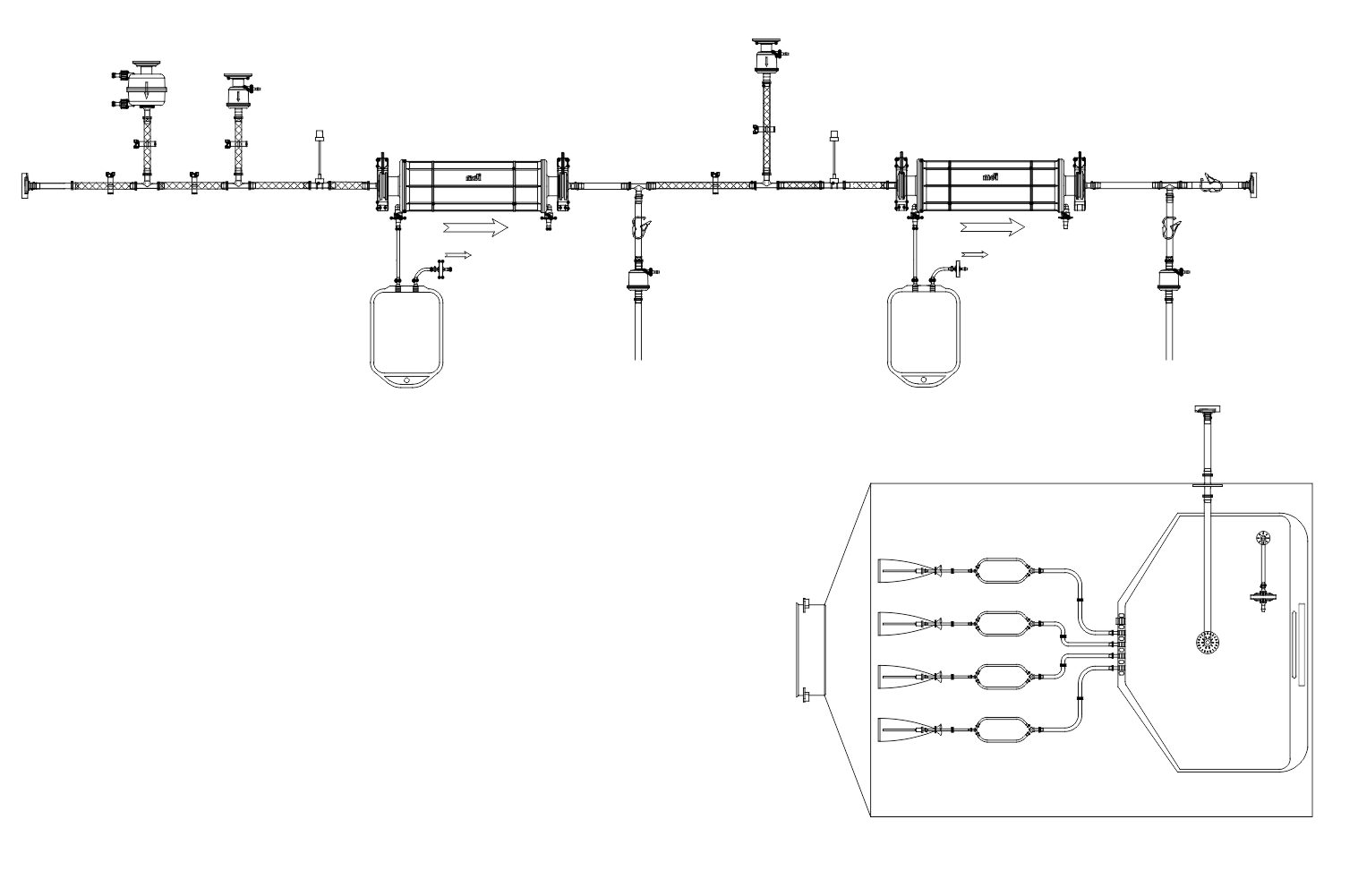
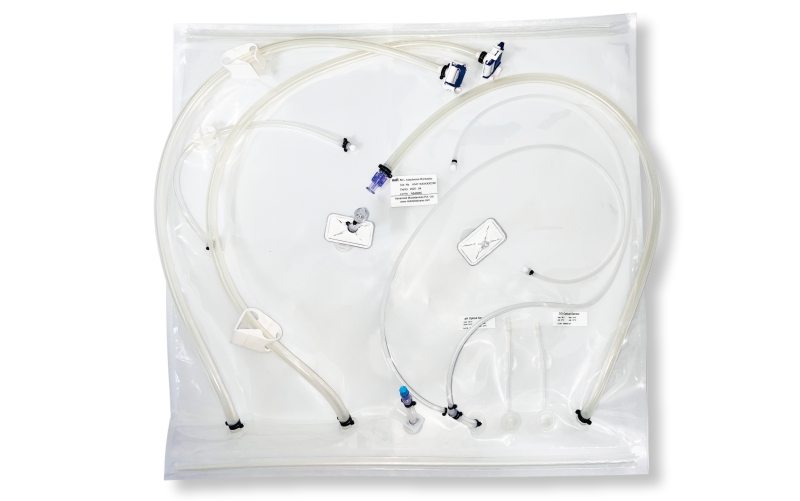
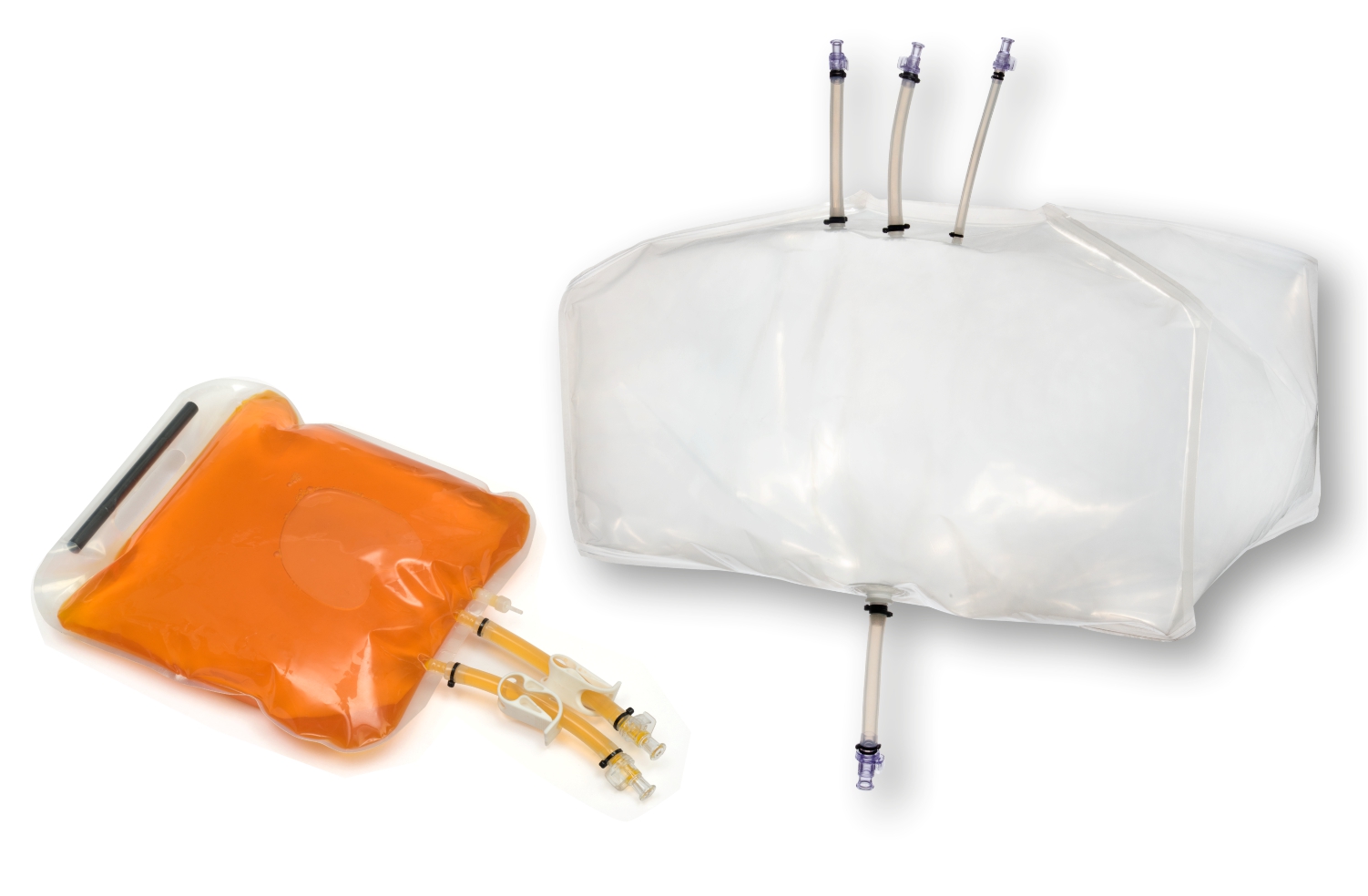
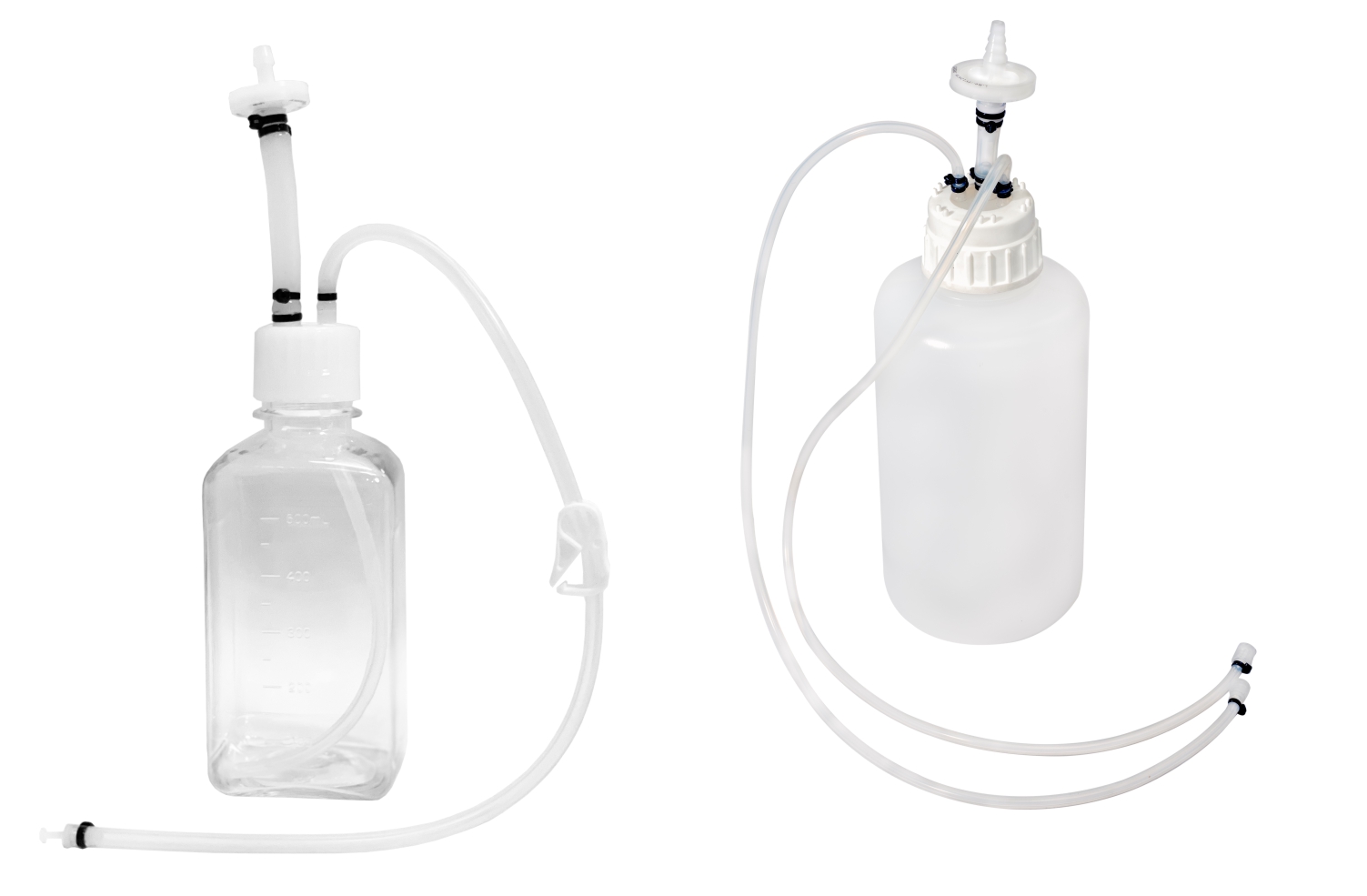
SUS have not only facilitated continuous processing but also enabled research scientists as well as process owners to work with different molecules such as antibodies, proteins, vaccines etc in the same facility. All this has resulted in faster lab to the market movement of the new biopharmaceutical drugs contributing to overall growth of the industry. However, SUS involve a wide variety of polymeric components such as membrane filtration devices; bags; connectors; tubing; and fittings; and range from simple transfer systems to complex disposable filling lines.
mdi offers a wide range of gamma irradiated SUS for various critical applications in biopharmaceuticals. These range from:
- Storage and transfer systems for media, buffers and drug substances
- Sampling manifolds for bioreactors and process intermediate reservoirs
- Complex disposable filling lines complete with PUPSIT (Pre-use/ Post Sterilization Integrity Testing)
All the key components used in mdi SUS are produced in house and are deeply characterized and validated for:
- Integrity
- Microbial retention/ingress
- Sterility
- Bacterial endotoxins
- Particulate Matter
- Biosafety
- Extractables/Leachables